Kingdom: |
Animalia Chordata Mammalia Artiodactyla Ruminantia Bovidae Caprinae Rupicaprini Capricornis |
-
Capricornis swinhoei [Gray, 1862].
- Citation: Ann. Mag. Nat. Hist., ser. 3, 10:320.
- Type locality: Taiwan.
- Citation: Ann. Mag. Nat. Hist., ser. 3, 10:320.
The taxonomic record (above) is taken from Wilson and Reeder (1993). The serows, while often assigned to the genus Capricornis, were combined into the genus Naemorhedus by Groves and Grubb (1985). The most recent taxonomic revision (Wilson and Reeder, 2005) has restituted Capricornis to a full genus in its own right. The Formosan serow is considered by some authors to be a subspecies of the Japanese serow (Capricornis crispus), although according to Groves and Grubb (1985) C. swinhoei is a distinct species.
Physical Characteristics
| Reported measurements for Formosan serow (Capricornis swinhoei) | ||||
| Source | Adult Weight | Head & Body Length | Shoulder Height | Tail Length |
| Sheng Helin et al., 1999 | - | 80-114 cm | - | - |
| Ito, 1987 | - | 85-92 cm | 60 cm | - |
| Nowak, 1991 For Capricornis |
50-140 kg | 140-180 cm | 85-94 cm | 8-16 cm |
| Smith and Xie, 2008 | 17-25 kg | 80-114 cm | 50-60 cm | 7-12 cm |
| Wang and Chen, 1981 | 30 kg | - | - | - |
The only conspicuous marking is a large square "bib" of creamy white to golden hair - this runs from the chin, under the jowls, and onto the upper throat (the back of the neck may have brownish yellow to light brown spots) (Lue, 1987; Sheng Helin et al., 1999). The ears are large (Ito, 1987). A pair of suborbital (or preorbital) glands is present in front of the eyes, a characteristic which distinguishes the serows (genus Capricornis) from the gorals (genus Naemorhedus) (Groves and Grubb, 1985; Ito, 1987). A diagnostic skull characteristic of this species is the large, highly trenchant ridge on the upper edge of the lacrimal fossa (Groves and Grubb, 1985).
Both sexes possess sharp horns that curve slightly backwards (Sheng Helin et al., 1999). Although no horn lengths have been reported specifically for C. swinhoei, Nowak (1991) described the horns of Capricornis as being 15.2-25.5 cm in length, with narrow transverse ridges on the basal three-quarters. Due to the small relative size of C. swinhoei, the horns are likely at the lower end of (or less than) this range of lengths.
Reproduction and Development
Ecology
Little is known about the current population structure, although the high altitude areas of Yu-shan National Park are believed to support an average density of 22 serows per km² (K. Y. Lue in Chiang and Pei, 2008).
No native predators of C. swinhoei remain on Taiwan, although the clouded leopard (Neofelis nebulosa) may have been a prime predator in forests prior to its extirpation (Lue, 1987). The Formosan serow may compete with Formosan sambar (Cervus unicolor swinhoei) and Formosan muntjac (Muntiacus reevesi micrurus), the two other large herbivores on Taiwan (Lue, 1987). However, recent studies by Chiang (2007, in Chiang and Pei 2008) suggest that such competition may be mitigated by habitat segregation.
The Formosan serow is an intermediate feeder, browsing on juvenile parts of conifers and also feeding on grasses and shrubs growing on disturbed slopes in the early stages of succession (McCullough in Lue, 1987; Lue, 1987). From interviews with aborigines, Lue (1987) reported that preferred food plants include Urtica fissa, Elatostema edule, Anisogonium esculentum, Begonia laciniata, Polygonum chinensis, Chamabainia cuspidata, Mussaenda parviflora, Perrottetia arisanensis, and Pellionia arisanensis. Although poisonous, Urtica fissa does not seem to harm Formosan serows (Lue, 1987). At the Taipei Zoo, on a diet consisting of pellets, carrots, fresh Napier grass, and soybean curd pomace, each animal ate approximately 2.3 kg per day amounting to a daily intake of 1,940 kcal (Wang and Chen, 1981).
Behavior
C. swinhoei is reported by Smith and Xie (2008) to be crepuscular, while Sheng Helin et al. (1999) suggest that the species is most active throughout the night and in early morning. However, Chiang and Pei (2008) report that in highly remote areas with minimal human activity, diurnal activity is prevalent (74% of the activity budget), with most activity occurring 3-4 hours after sunrise and again before sunset. Among recently captured individuals at the Taipei Zoo in Taiwan, most of the food provided was consumed in the evening and at night (Wang and Chen, 1981).
Several captive individuals were observed by Chen (1987) rubbing trees and other vertical structures with their preorbital glands and horns. This marking behaviour was closely related to social rank, with the most dominant individuals marking the most frequently; in the wild, this behavior is likely used for delineating territories. Individuals also utilize specific defecation middens (Chen, 1987).
When irritated, Formosan serow will stamp their forefeet, an action they also do as a warning (Wang and Chen, 1981; Chen, 1987). A shrill alarm call may accompany the stamping, sounding like a mixture between a whistle and a scream (Wang and Chen, 1981). Formosan serow are excellent climbers and strong jumpers, easily clearing obstacles two meters in height (Wang and Chen, 1981). If pressed, Formosan serows will climb trees, and at the Taipei Zoo have been seen in branches up to three meters above the ground (Chen, 1987).
During courtship, the male will pursue the female, gently touching her with his horns or using his front legs to intermittently touch the female's belly and hips (Chen, 1987). Aggressive encounters involve individuals facing each other with horns lowered (Chen, 1987).
Genetics
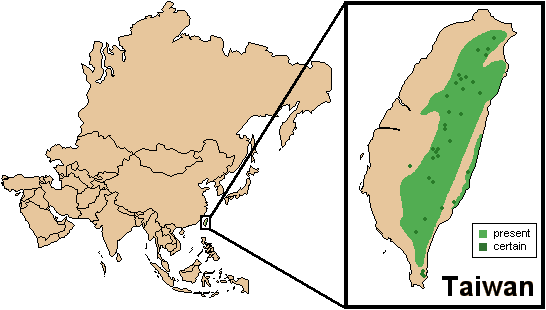
Distribution
Countries: Taiwan, Province of China (Chiang and Pei, 2008).
Conservation Status
Remarks
Formosa is an archaic name for the island of Taiwan. Although the name Taiwanese serow is used by some authorities, Formosan serow remains the preferred title. Serow ("suh-ROE") is a name used by the Lapchas of Sikkim in the Himalayas to refer to C. sumatraensis, although this name is now used to refer to all members of the genus Capricornis (Gotch, 1995).
Capra (Latin) a she-goat; cornu (Latin) the horn of an animal; hence "Capricornis" implies the presence of goat-like horns. R. Swinhoe FRS (1836-1877) was at one time in the British Consular Service in China (Gotch, 1995).
-
- Local names
- "Taiwan Leiling" [Phonetic Chinese] (Smith and Xie, 2008)
-
- German
- Formosa Serau




